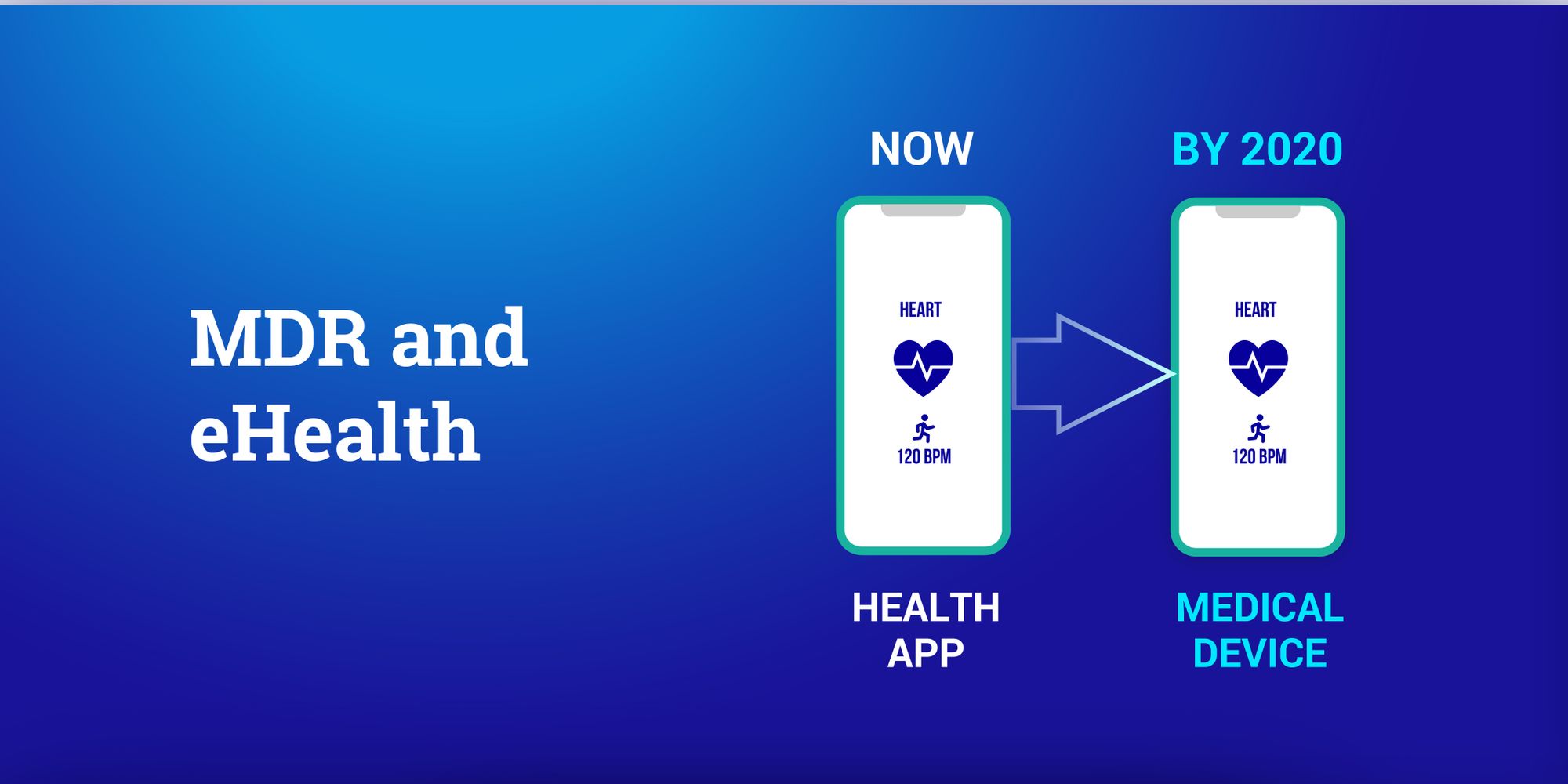
eBooks for health and medical app security
Get the latest on Health and Medical App Security - and learn how you can make your Apps secure. From 2014 onwards our team was working on these topics and prepared some eBooks and video recordings for you.