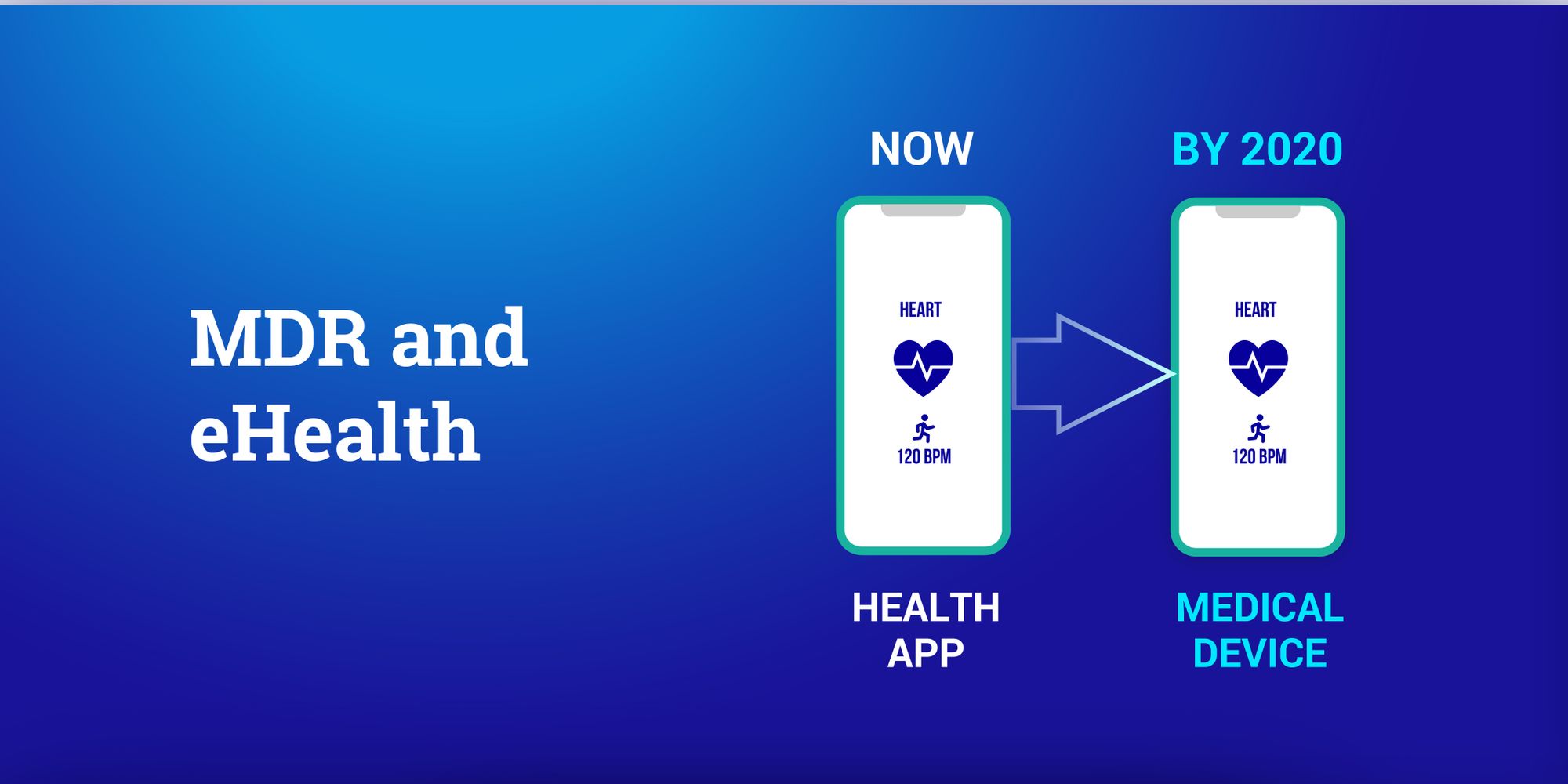
The MDR (Medical Device Regulation) will be enforced from May 2020. Throughout the past year we received number of questions from digital health innovators on how it will affect their project development timeline. all questions are quite similar, therefore, we are putting this session to answer most urgent questions of yours.
In this webinar, we look at the implications of MDR, why GDPR compliance is essential and how all this impacts your project timeline.
Why does MDR and GDPR compliance matter?
The MDR (medical device regulation) will affect many developers of eHealth applications. Software is explicitly covered and can even receive the highest risk rating (Class III). This means you need to understand how to become compliant. We discuss this in more detail in our new MDR eBook.
Download our eBook on building MDR compliant applications
Download nowHow hard is MDR compliance?
Building applications that comply with all relevant regulations (MDR, GDPR, etc.) is tough. Mandatory requirements include legal, security, quality and classical technical tasks. Most eHealth application developers don't have all the relevant knowledge. And this effort may seem like a distraction from value-add activities, i.e. designing your product, understanding customers, marketing, revenues and investments. However, if you don't get MDR certification, you won't be able to distribute or sell your application in the EEA.
Register for webinar
If you need more information, join us in September for a webinar on this topic. We will explain some of the tools and technologies that can speed up MDR and GDPR compliance. Our CEO, Dr. Jovan Stevovic will be presenting alongside Matteo Gubellini, the Co-founder & VP of Regulatory Affairs/Chief Regulatory Officer at SoftComply.