France has introduced a fast-track market access pathway for DTx and digital health products, adding to similar schemes in neighboring EU countries Germany and Belgium.
The early coverage process that includes DTx and medical telemonitoring solutions has been approved on March 31, 2023, by the Ministry of Health and Prevention.
The early coverage process is called PECAN (prise en charge anticipée des dispositifs médicaux numériques), and it introduces (like the German DVG/DiGA) a one-year special coverage by the French health care system for “sufficiently” mature solutions.
This means that the process allows companies to be reimbursed while they finalise the demonstration of their clinical and/or organisational benefits.
If your DTx is approved, you will be part of the List of Products and Services reimbursable by the French Health Insurance Fund, which covers 60 million people.
How does the new reimbursement program work, and which products are eligible for coverage?
Who can apply to PECAN?
PECAN allows temporary coverage by the health insurance of the following DMD (Digital Medical Device):
- Therapeutic digital medical devices (DTx).
- Remote medical monitoring activities.
The requirements are that companies provide the data necessary to evaluate the medical device and that the latter already complies with safety requirements.
To apply to the pathway, startups, and companies should meet some initial pre-requisites:
- Being innovative, particularly in terms of clinical benefit or progress, according to the first available data and taking into account any relevant comparators;
- Having a valid medical CE mark (for the specific intended use), whatever the risk class of the device is. Product documentation and labeling must be translated into French.
- Being able to provide evidence of the benefits of the solution within the timeframe allowed for the HAS to decide on the benefits (and before the end of the derogation period);
- Complying with the MIS interoperability and security guidelines, the Agence du Numérique en Santé established to guarantee the exchange, sharing, security, and confidentiality of patient health data.
- Not yet reimbursed in France: only new solutions can apply to PECAN. DTx already reimbursed in France can’t apply to this fast-track.
At this point, it is important to remember that significant software changes may require a re-certification by the responsible authority.
Class I products need to fulfill the same guidelines as higher-risk classes but do not need to involve a notified body.
What’s the process like?
The Fast Track Reimbursement Path
Now that we have cleared the prerequisites, let’s go through the main steps you need to do to apply to the Fast-Track in France. First, remember that the temporary coverage is provided for one year.
- Submit the technical documentation and initial clinical evidence (to the Ministry of Health and Social Security and CNEDiMTS via the HAS EVATECH platform.)
◾️ DMD’s proof of compliance on the ANS’ Convergence platform
◾ ️Certification of Interoperability and Security
◾️ Dossier about medical and technical specifications
◾️ Dossier about economic’s information.
2. Review of the application.
◾ ️Receive the opinion of the CNEDiMTS once it has evaluated the clinical or organisational benefit of your digital solution.
◾️ Receive the certificate of compliance with the interoperability and security framework from the ANS.
3. Your solution is eligible for early reimbursement.
◾️ Receive the decision by order of the competent authority (the Ministers of Health and Social Security).
◾️ Your DMD or remote monitoring activity is reimbursable for a one-year, non-renewable period at a predetermined flat rate.
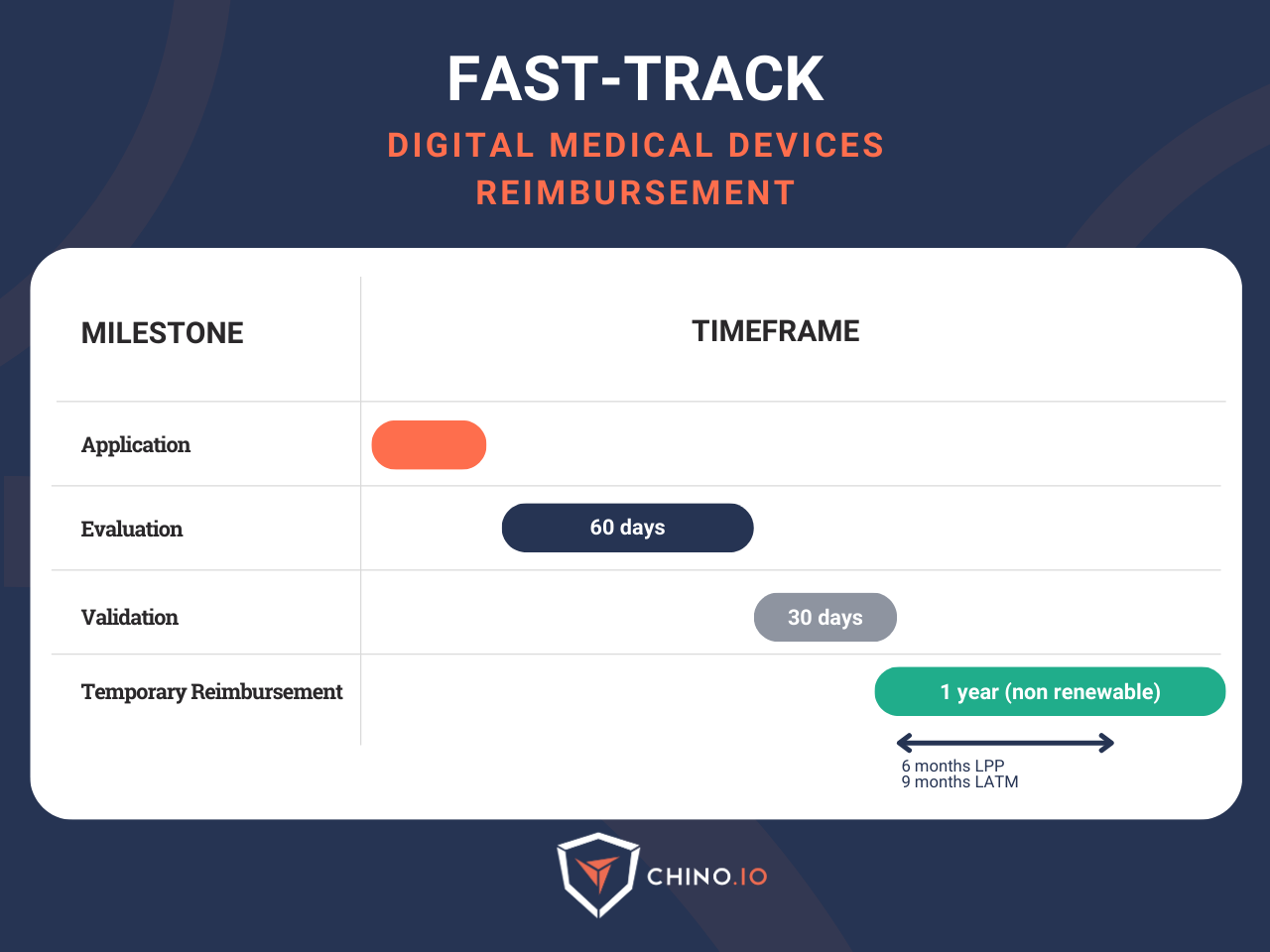
The complete evaluation and validation process lasts up to 90 days (60 days for the assessment by the Digital Health Agency (ANS) and the National Authority for Health (HAS) + 30 days for the final decision by the French Ministry of Health).
Remember that once the early coverage is established, you have 6 months to apply for the registration of the devices in the LPPR (List of Reimbursable Products and Services).
Ok, we have been writing about DTx, but what about medical telemonitoring solutions? The process is similar but not exactly the same: manufacturers must apply for reimbursement through LATM for 9 months from the start of the early coverage.
Standard Reimbursement Path
You can access the Standard Reimbursement scheme once the one-year fast-track pathway is over.
The permanent reimbursement usually lasts several years and is renewable, so don’t miss this chance.
Let’s go through the 3 main steps you need to follow to submit the standard reimbursement:
- Submit your application for inclusion in the standard system: If your solution is a therapeutic digital MD, you have 6 months from the fast-track reimbursement period to apply for registration on the list of products and services qualifying for reimbursement (LPPR) as a brand name. But, if your solution is a remote monitoring activity, you have 9 months to apply for registration on the list of medical telemonitoring activities (LATM).
- Review of your application: the CNEDiMTS (HAS) will review the evidence of clinical benefits or improvements in how care is organised.
- Your solution becomes eligible for regular reimbursement: if approved, your DTx (or telemonitoring solution) will be added to the list of reimbursed products (LPPR or LATM) for several years and renewable.

Want to know more about the other main reimbursement pathways available? Check out our blog article about DTx and how to get reimbursed in the EU, UK, and US!
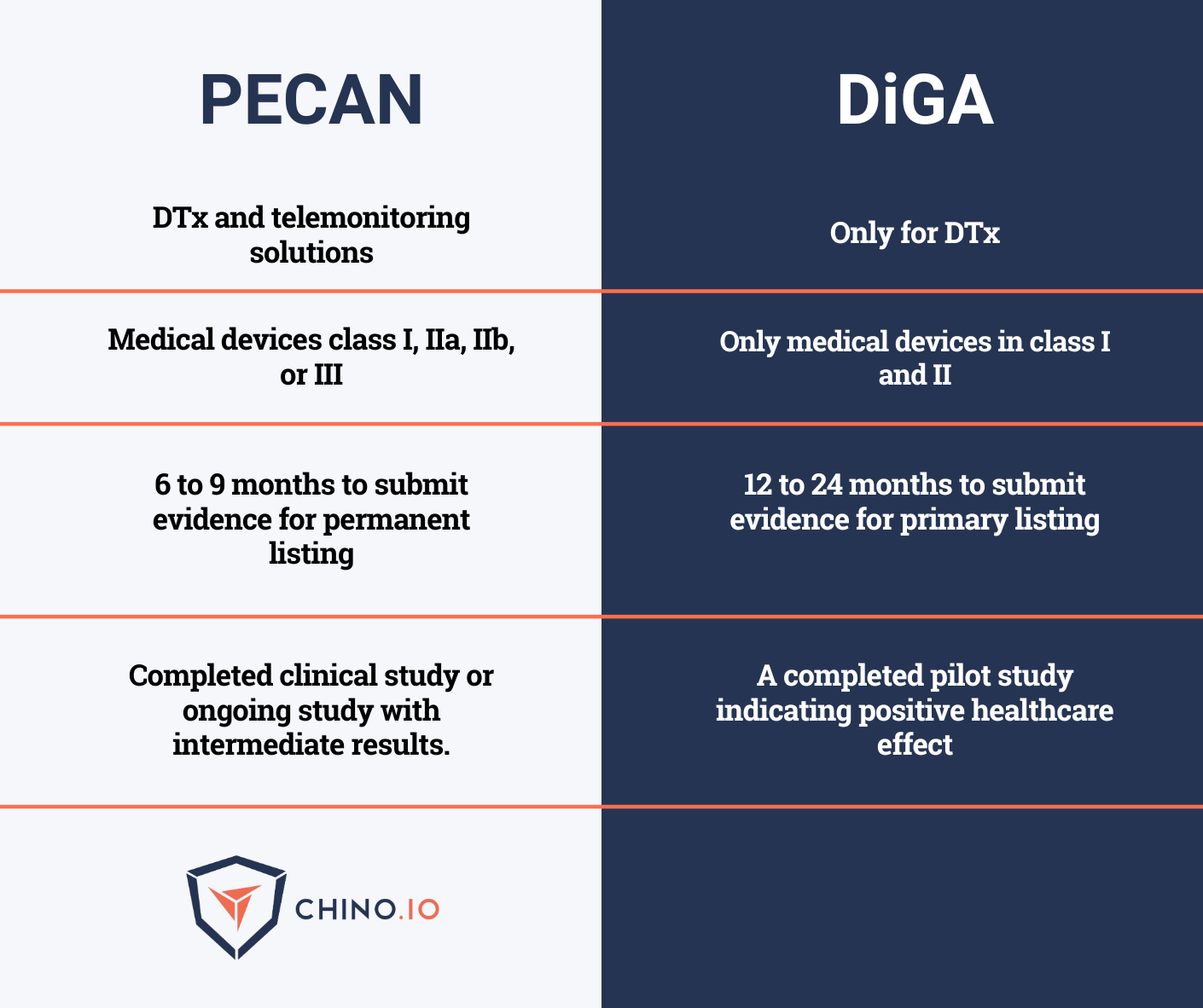
What’s the difference between DVG/DiGA and PECAN?
Luckily, there are many similarities between the requirements for DiGAS and PECAN.
If you have a DiGA, you have quite every requirement to submit your application to PECAN as well.
Let’s see a comparison between the two reimbursement pathways:

Data protection requirements
Any DMD (as well as DiGA) will have to meet strict requirements relating to data protection, data security, and quality.
Firstly, you will prove that your application is GDPR compliant.
Here, we refer to Requirement 103 of the Benchmark Interoperability and Safety of Digital Medical Devices, stating that devices must comply with the legislative and regulatory provisions according to:
- Law No. 78-17 of January 1978 on information technology, files, and freedom
- GDPR compliance, in particular, requirements of Article 28 (processor), Article 32 (security of processing), and Article 35.
Concretely, PECAN will require DMDs to submit a Declaration of conformity with the General Data Protection Regulation Data (see annex 4.2)
Main requirements outlined in the declaration of conformity:
- Conduct a DPIA (Data Protection Impact Assessment) for your solution.
- Implement state-of-the-art security measures.
- All your sub-contractors or processors regulated by a data processing agreement, and ensure that they are GDPR compliant.
Non-compliance with the data protection requirements will expose the DMDs to sanctions by CNIL (French Data Protection Authority).
As Chino.io, we help companies in getting their DTx reimbursed
Some tips for DTx companies that may be looking to apply
- Implement explicit consent: personal data can only be processed if there is a clear legal basis. For DTx reimbursement programs, the legal basis is not necessarily relying on “explicit consent, which is necessary typically for handling sensitive data in classical digital health products. However, for some data processing, you need a clear affirmative action and an appropriate consent form in your app (make sure you don’t miss our article about consent).
- Provide a clear and easy-to-understand Privacy Policy: every solution must be accompanied by a data protection declaration, which must contain specific information such as the purpose of your product, information about the manufacturer, who has access to the data, the right to revoke the consent, etc.
- Demonstrate you do not transfer data to the USA: under DVG, data processing in the US or any other Third Country is very problematic and mostly not allowed unless the data is encrypted with an external Key Management System in the EU (from an EU provider). It’s not clear yet what will be the ANS position, but since both schemes are based on the GDPR, it’s a good idea to take a cautious approach toward the choices made around cloud providers and services.
How Chino.io can help to get you ready.
The PECAN program is another huge opportunity for digital health solutions (60 million people market).
As you may have noticed, there are many similarities (but also differences) among different reimbursement schemes in the EU, UK, and US (data privacy, security, and GDPR or HIPAA compliance).
Thus, it’s fundamental to work with the right partners to implement and evolve your compliance system that can enable you to access all markets.
Below are the four leading suggestions that you may benefit from when approaching the go-to-market in France (and EU countries as well):
- Start with GDPR basics from day 0. National regulations like PECAN and DiGAV bring additional data security, privacy, and overall data management conditions. However, the starting core is always GDPR; getting your data handling sorted out from the start is critical. In addition to the reimbursement process, users, partners, and investors are increasingly aware of privacy risks and will demand high compliance standards from you. If you don’t get compliance right, you can expect a significant impact on client acquisition, partnership deals, and user retention.
- Surround yourself with trusted partners: the regulatory framework is evolving very fast. This means more challenges but also more opportunities and tougher competition. Time-to-market and efficiency will be the essence to compete with lower price points and innovation, so consider collaborating with trusted partners that can maximise your efforts and support you in your journey with strategic contributions.
- Plan a clear roadmap: if your goal is to target a process such as the PECAN or the DVG’s Fast-Track, the first 12 months will be your initial main focus, and having a clear roadmap where all aspects, including compliance, are covered, are a deciding factor.
Be sure to prevent months-long approvals and certifications (some may take up to 6-12 months!) by planning ahead. - Choose the right cloud provider: reimbursement rules and the EU healthcare system, in general, are very strict regarding using cloud providers. For example, the DiGAV states that personal data can only be processed in a 3rd country if there is an adequate decision in place. After the invalidation of the previous adequacy decision on the EU-US Privacy Shield, the US is still considered TODAY a Third country without an Adequacy Decision according to GDPR (here is an article about this topic). This makes it very complex to rely on US processors to handle your application and the data you collect. Chino.io can help you out defining a compliant architecture for your DTx, taking into account the key reimbursement schemes for your business.
If you want to know more about this reimbursement scheme, check out the official PECAN reference.
Chino.io, your trusted compliance partner
Working with experts can reduce time-to-market and technical debt and ensure a clear roadmap you can showcase to partners and investors.
At Chino.io, we have been combining our technological and legal expertise to help hundreds of companies like yours navigate through EU and US regulatory frameworks enabling successful launches and reimbursement approvals.
We offer tailored solutions to support you in meeting the GDPR, HIPAA, PECAN, DVG, or DTAC mandated for listing your product as DTx.
Want to know how we can help you? Reach out to us and learn more.





